News
UK NEQAS Feature in May Edition of Pathology in Practice
We’re proud to announce that we feature in the May edition of Pathology in Practice. This prominent appearance highlights the exceptional work UK NEQAS undertakes and is introduced with a striking blue front cover.
Readers can find UK NEQAS throughout the publication on pages 3, 7, 11, 15, 16, and 17. The main article, entitled “UK NEQAS: Delivering Far More Than External Quality Assessment,” begins on page 15. In this piece, Liam Whitby, President of UK NEQAS, elaborates on why UK NEQAS is considered a global leader in External Quality Assessment (EQA).
For those who haven’t yet seen the publication, the main article is accessible by clicking on the link above. This feature not only underscores the achievements of UK NEQAS but also provides insightful perspectives on the critical role of EQA in pathology.
Recommended Cells Markers in LPD Diagnosis
UK NEQAS LI contributed data to a Delphi Poll conducted by Dr Iman Qureshi. The poll sought to gather information from respondents on recommended cell markers in lymphoproliferative disorder diagnosis. The findings were published in a poster which can be found below;
Recommended cells markers in LPD diagnos[...]
Adobe Acrobat document [463.1 KB]
The National Measurement Laboratory and UK NEQAS for Leucocyte Immunophenotyping announce collaboration to improve quality assessment schemes for Chronic Myeloid Leukaemia Minimal Residual Disease testing
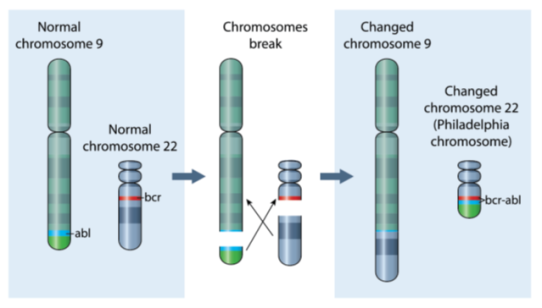
The National Measurement Laboratory (NML, London, UK) and UK NEQAS for Leucocyte Immunophenotyping (UK NEQAS LI, Sheffield, UK) are pleased to announce a collaborative project aimed at improving the measurement of the BCR::ABL1 fusion gene, the causative genetic mutation in chronic myeloid leukaemia (CML) which occurs during a chromosomal rearrangement leading to formation of the Philadelphia chromosome (Figure 1). Quantification of the BCR::ABL1 fusion gene transcript (messenger RNA) is a critical factor in the management of patients with CML, following treatment with targeted inhibitors of the BCR::ABL1 protein (TKIs), or stem cell transplantation. The collaboration aims to explore the potential of reverse-transcription digital PCR (RT-dPCR) to enhance the accuracy and traceability of BCR::ABL1 quantification in current External Quality assessment (EQA) schemes.
Figure 1: Formation of the BCR::ABL1 fusion gene following reciprocal translocation events between chromosomes 9 and 22.
The project, ‘Feasibility of a Digital PCR-Based Reference Measurement Procedure’, aims to develop an RT-dPCR-based Reference Measurement Procedure (RMP) for BCR::ABL1 Quantification. Clinical analytes are often standardised through a calibration hierarchy involving both Reference Materials and RMPs, which enable traceability of diagnostic assays and ultimately, patient results to higher-order standards. To act as an RMP, superior accuracy is necessary, therefore this project will assess potential technical sources of bias as well as define the precision of BCR::ABL1 measurements. The candidate RMP will be applied to EQA samples from previous rounds to establish comparability with the current consensus-based approach.
The NML hosted at LGC, is an international leader in the measurement science supporting sectors such as health and diagnostics. The Molecular and Cell Biology team within NML has state-of-the-art
facilities (Figure 2) and unparalleled expertise in high accuracy quantitative nucleic acid measurements. NML was one of the first laboratories in UK to establish dPCR and is at the forefront of
applying the approach to health-related challenges such as cancer genomics, liquid biopsy, infectious disease testing and characterization of advanced therapeutics. "Recent advances in dPCR have been
applied in a number of fields to enable standardisation of molecular methods,” says Prof. Jim Huggett, Science Fellow at NML. “This is a timely collaboration that will hopefully underpin the accuracy
of CML testing, ultimately leading to better patient outcomes”.
Figure 2: NML Molecular Biology. The laboratory is home to a number of dPCR instruments including the QX200 Droplet Digital PCR System (Bio-Rad) and QIAcuity-4 (QIAGEN) as well as RT-qPCR and sequencing instrumentation.
Liam Whitby, Director at UK NEQAS LI said, "We are extremely excited about this collaboration. By joining forces with NML, we aim to set a new standard in BCR::ABL1 quantification, enhancing the quality of patient care in CML management." With over 15 years of commitment to external quality assessment for BCR::ABL1 Quantification in CML, UK NEQAS LI has been at the forefront of advancements in the field of leukaemia diagnostics. Their BCR::ABL1 Quantification programme, with over 300 participants globally, is specially designed to meet the clinical requirements for CML management and is aligned with the WHO International Scale.
The initial validation performed during the project will provide a foundation for the development of a published, openly available RMP for BCR::ABL1, which has the following potential benefits:
• Facilitate pre-assignment of values to EQA samples, enabling more robust QC prior to initiation of an EQA round
• Allow a harmonised scoring approach for all participants, avoiding potential bias in the consensus values due to their association with the most commonly used molecular methods
•Ensure the EQA programme has the highest levels of metrological traceability to the BCR::ABL1 International Scale, providing direct calibration to the WHO primary standards
•Embed EQA in a metrology framework, ensuring its long-term stability and comparability with other EQA providers
•Availability of the method for value-assignment of other BCR::ABL1 standards produced by commercial providers and academic consortia
•Reduced variation in clinical testing of BCR::ABL1, ensuring the reproducibility of minimal residual disease measurements between laboratories.
Over time, this work will ultimately build on standardisation efforts from the previous two decades and underpin robust clinical decision making in CML patient management.
For more details about the project, please contact Alison Devonshire, Principal Scientist NML (alison.devonshire@lgcgroup.com) and Stuart Scott, Centre
ManagerCentre Manager at at UK NEQAS LI (Stuart.scott@ukneqasli.co.uk)
Virtual Flow Cytometry Data Analysis Course Spring 2024
The RMS is holding an interactive virtual course on flow cytometric data analysis. The Research Module will be held 26 - 29 February 2024, plus one optional additional Clinical Module on 4 March 2024.
To book your place on the course or to find more information please follow the link below;
Webinar: The Evolving Landscape of Genetic Testing in MPNs
In the 2024-2025 registration period, UK NEQAS LI will be consolidating the JAK2 p.Val617Phe (V617F) Mutation Status and
Myeloproliferative Neoplasms Diagnostic Testing programmes to better reflect current laboratory practise.
In order to promote these changes we’ll be hosting a webinar on Tuesday 9th January 2024. We’re delighted to have Prof. Claire Harrison (Guy's and St. Thomas' Hospital, UK) who will be discussing
‘Clinical Implications and Advances in Genetic Testing in MPNs’. We will also have Dr Hazel Clouston, the programme lead for the JAK2 p.Val617Phe (V617F) Mutation Status and the Myeloproliferative
Neoplasms Diagnostic Testing programmes at UK NEQAS LI, discussing the forthcoming changes to the programmes. Finally, we’ll have a Q&A session where Claire and Hazel will be joined by
Professor Nicholas Cross (University of Southampton and Wessex Regional Genomic Service, UK) and Dr Zi Ng (Royal Perth Hospital, Australia).
For further details and how to register please go to the follwoing website: https://ukneqas.org.uk/events/the-evolving-landscape-of-genetic-testing-in-mpns/
New for 2024 - 2025
As part of the continuous development of our EQA/PT provision we will be introducing a number of updates to our flow cytometry and molecular haematology programmes in 2024-2025.
The re-registration period for the 2024-2025 year opens on the 1st February 2024.
Please see the molecular and flow cytometry pages on the website for further details.
New European LeukemiaNet Laboratory Recommendations for Chronic Myeloid Leukemia Released
We're pleased to share that the European LeukemiaNet has recently updated their laboratory recommendations for the diagnosis and management of Chronic Myeloid Leukemia (CML). These guidelines are another important step forward in standardising care for patients with CML. It's heartening to note that some of our own publications at UK NEQAS for LeucocyteImmunophenotyping (UK NEQAS LI) were considered in the development of these recommendations. We're honoured to have had the opportunity to contribute to the discourse in this area.
https://www.nature.com/articles/s41375-023-02048-y
Acknowledging Dr Debbie Travis
Special mention to Dr Debbie Travis from our team, who assisted with contributions focused on BCR::ABL1 TKD variant (mutation) testing and has been acknowledged by the authors.
The Importance of Updated Guidelines
These revised recommendations offer a valuable resource for healthcare providers involved in the diagnosis and management of CML, and we're glad to be a part of a community that continually seeks to improve and standardise patient care. Feel free to share these updates with your network and here’s to ongoing progress in the fields of laboratory medicine and leukaemia genetics.
Latest Publication from UK NEQAS LI
We’re excited that the final version of our paper ‘Assessment of acute myeloid leukemia molecular measurable residual disease testing in an interlaboratory study’ is now available: https://ashpublications.org/bloodadvances/article/7/14/3686/494977/Assessment-of-acute-myeloid-leukemia-molecular
We’re already building on this work by looking at ways we can improve molecular AML MRD testing as part of our Knowledge Transfer Partnership collaboration with the National Measurement Laboratory, National Institute of Biological Standards and Controls and the UK Accreditation Service: https://www.npl.co.uk/leaders-in-healthcare-science
The talks from our webinar are still available on Youtube: https://www.youtube.com/playlist?list=PLCRdBttSCjlB2I6KR6toBSvSnz7-Ue1iI
UK NEQAS IVDR UPDATE
The European Union In Vitro Diagnostics Regulation [Regulation (EU) 2017/746 (EU IVDR)] was implemented on the 26th of May 2022. A statement of the application of the IVDR to external quality assessment (EQA) services can be found here.
Best Practice and Standardisation of Molecular Measurable Residual Disease Testing in Leukaemia webinar
UK NEQAS LI are delighted to say that the talks from our Best Practice and Standardisation of Molecular Measurable Residual Disease Testing in Leukaemia webinar are now available at the following link: https://www.youtube.com/channel/UC-nD3u65dTAo09gNBeFTeWA
The webinar is the first phase of a knowledge transfer partnership assessing the need for further standardisation of measurable residual disease testing in AML selected by the Chief Scientific Officer as part of their 2022 programme
https://www.npl.co.uk/leaders-in-healthcare-science - current projects
Knowledge transfer partnerships are collaborations at a senior level of NHS scientists with partner organisations including the United Kingdom Accreditation Service (UKAS) and partner organisations that collectively deliver the UK's National Measurement System (NMS); The National Measurement Laboratory (NML) hosted at LGC; The National Physical Laboratory (NPL) and The National Institute for Biological Standards and Control (NIBSC). By facilitating early interaction and knowledge exchange, the programme aims to speed up the identification and dissemination of high value new approaches to improving patient outcomes and increasing efficiency, while also promoting economic growth and inward investment in the Life Sciences.
The objectives of the webinar were to:
- To improve basic knowledge of molecular measurable residual disease (MRD) testing
- To share best practice for MRD testing
- To look at the current use of acute myeloid leukaemia (AML) MRD testing and assess what more can be done to reduce intra and inter laboratory variation
NEW FOR 2023-2024
As part of the continuous development of our EQA/PT provision we will be introducing a number of updates to our flow cytometry and molecular haematology programmes in 2022-2023.
The re-registration period for the 2023-2024 year opens on the 1st February 2023.
Please see the molecular and flow cytometry pages on the website for further details.
Publication from UK NEQAS LI
UK NEQAS LI has recently assessed the implementation of the National Institute for Health and Care Excellence (NICE) haematological cancers improving outcomes guidelines (NG47) across Specialist Integrated Haematological Malignancy Diagnostic Services in England. Read about the findings here:
https://jcp.bmj.com/content/early/2022/05/03/jclinpath-2021-208075
Publication from UK NEQAS LI
UK NEQAS LI was recently part of an international harmonized study for data analysis in the evaluation of multiple myeloma residual disease by high sensitivity flow cytometry. Read about the findings here:
https://onlinelibrary.wiley.com/doi/full/10.1002/cyto.b.22053
Publication from UK NEQAS LI
Read the latest publication from UK NEQAS LI that has assessed the impact of the 2017 European LeukemiaNet recommendations on FLT3 allelic ratio calculation and reporting in AML:
https://onlinelibrary.wiley.com/doi/10.1111/bjh.18023
Connect with us
UK NEQAS for Leucocyte Immunophenotyping (UK NEQAS LI) now has a Facebook page and a Twitter account. We will be posting important messages to these accounts relating to our schemes so please like or follow us, respectively.
We hope that these feeds will act as a useful eductational resource for participants and collaborators alike, collating interesting papers, conference info, videos and news stories in haemato-oncology. As we develop our online presence we hope to publish some of our own educational content. If you have any feedback or ideas as to content you would like us to include, please do not hesitate to get in touch.